Solutions
Central Lab Services
At Medicover Integrated Clinical Services, we offer end-to-end global Central Lab for clinical trials, providing Sponsor and CRO companies with focused and agile approach ensuring successful delivery of clinical research projects of all sizes and complexity.



One-stop-shop Global Central Lab Services


Medicover Integrated Clinical Services is a leading provider of comprehensive central laboratory services for clinical trials, dedicated to supporting Pharma, Biotech, and CRO companies in their pursuit of medical advancements. With a global network of state-of-the-art laboratories, innovative technology, and a team of experienced professionals, MICS offers end-to-end solutions for clinical trial testing. Our services encompass a wide range of diagnostic disciplines, including pathology, hematology, molecular biology, and more, ensuring thorough and precise data collection. We are committed to delivering reliable, high-quality results in compliance with regulatory standards, while also providing efficient project management and tailored solutions to meet the unique needs of each trial. With MICS, as your central lab partner, you can trust in our expertise to streamline your clinical trial processes and accelerate the path to improved healthcare outcomes.
Contents:


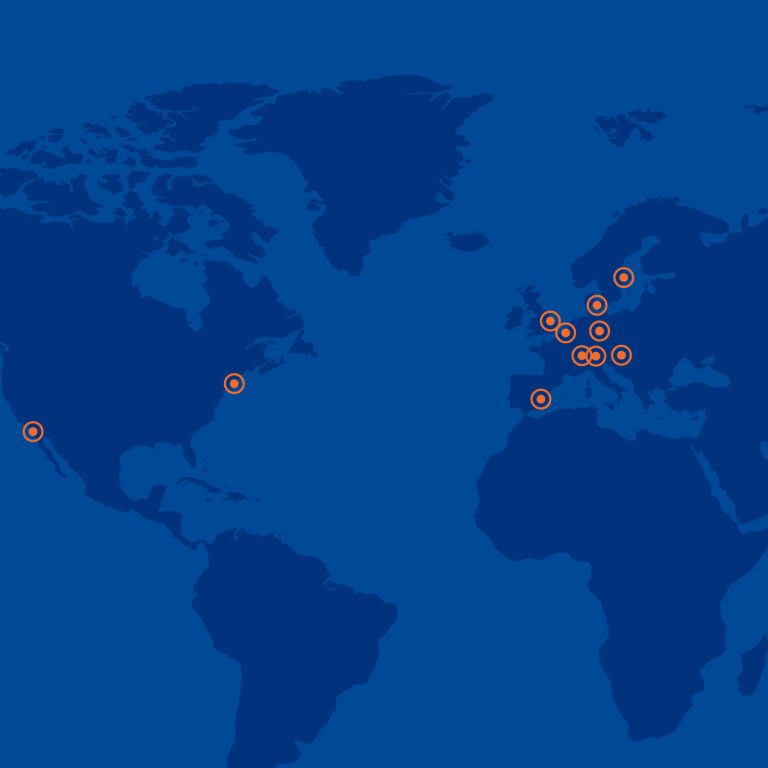
Global Central Laboratory Network
We offer unparalleled global coverage utilising not only our state-of-the-art proprietary proprietary laboratory facilities but also leveraging strategic partnerships with leading entities worldwide. The comprehensive network ensures that each clinical research projects, regardless of scale or complexity, receives the highest standards of quality and successful delivery.
At Medicover Integrated Clinical Services, our commitment to advancing healthcare knows no bounds, as we unite cutting-edge technology, global collaboration, and unwavering dedication to drive the success of clinical trials across diverse international landscapes.
Move the map
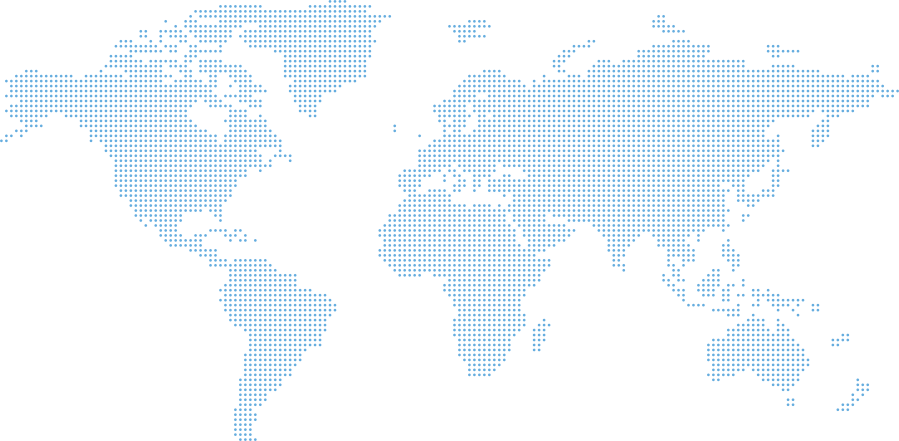
Europe
Germany — Berlin, Munich
Poland — Gdańsk, Łódź, Warsaw
Romania — Timisoara, Chiajna
Ukraine — Kiev
Bulgaria — Sofia
Georgia — Tibilisi
Moldova —Chisinau
Turkey — Istanbul
Serbia — Belgrade
Bosnia and Herzegovina — Sarajevo
Netherlands — Amsterdam
UK — Handforth
Argentina
Buenos Aires
India
Mumbai
Australia
Sydney
South Korea
Yongin-si
China
Shanghai
USA
Johnson City
Central Lab for clinical trials
Effective project management and continuous coordination are essential for the success of your clinical trials, ensuring precision and efficiency in execution. Explore the benefits of their integral role as part of our comprehensive laboratory management solution.

Laboratory Documentation
The clinical trial journey begins with an in-depth consultation where our team collaborates closely with yours to gain a comprehensive understanding of your trial’s objectives, timelines, and specific requirements. We thoroughly review the trial protocol, identifying critical laboratory parameters, sample types, and testing methodologies required for success ensuring the highest standards of quality and accuracy.
Laboratory Testing
Leveraging our extensive experience in clinical trials, we design a laboratory setup tailored to the unique needs of your study. This includes selecting the appropriate testing facility, equipment, and methods to respect the required timelines and effective turn-around time to prepare most cost-conscious offer and ensuring at the same time compliance with industry standards and regulatory guidelines.
Samples Management
We work with you to develop a robust sample management strategy. This encompasses everything from sample collection kits and labeling protocols to shipment and storage of samples. Our goal is to maximize sample integrity and traceability throughout the trial’s lifecycle.
Data Management
Ensuring compliance with international regulatory standards is necessary. Our team carefully examines your trial’s regulatory requirements and implements processes that align with Good Clinical Practice (GCP), Good Laboratory Practice (GLP), and Good Clinical Laboratory Practice (GCLP) guidelines. This guarantees the validity and acceptance of your trial data by regulatory authorities.
Lab Kits Production
We excel in producing custom collection kits tailored to your trial’s specific needs. Once kits are assembled, we design logistics processes, including secure transportation and storage, to guarantee that they reach your trial sites worldwide in a timely and controlled manner.
Project Management & Coordination
Our team provides comprehensive training and support to trial sites upon client request, ensuring that site staff are well-versed in sample collection, handling, and shipping procedures. This preanalytical step is crucial for maintaining the quality and consistency of the samples throughout the testing workflow in the trial.
Supplies and Samples Logistics
We conduct a thorough risk assessment to identify potential challenges and risks in the study setup process. Our proactive approach allows us to develop mitigation strategies, ensuring that any issues are addressed swiftly and efficiently to minimize disruptions to your trial.


Laboratory Services
Medicover Integrated Clinical Services offers access to a broad spectrum of diagnostic services including a wide array of laboratory tests, more than 8,000 parameters, in all major clinical pathology areas, ranging from routine to advanced tests and from prevention to monitoring of treatments.
Medicover Diagnostics regularly upgrades and invests in best-in-class laboratory equipment. Fully integrated automation from industry leaders Roche, Abbott, Beckman Coulter and Sysmex are implemented in the high throughput laboratory areas, each managing between 10,000-25,000 tests per day.
Advanced equipment in genetic testing features a NovaSeq™ High Throughput Next Generation Sequencer, a Hamilton based automation solution for DNA extraction, as well as a robust bioinformatics solution.
Using superior logistics, automation and an ever-growing portfolio of tests, we provide primary diagnostic testing as well as secondary opinion services globally.
Immunoassays
Cell-Based Assays
Histology
RT-PCR
Flow Cytometry
HPLC
Coagulation
Enzymatic Assays
Platelet Function Assays
Multiplex Assays


Main benefits
Being a Regional Central Lab, we benefit from reduced logistics costs, faster access to laboratory results, harmonized clinical data, and real-local support, all while tapping into a large patient population in Central and Eastern Europe.
Main features
Discover why Medicover Integrated Clinical Services is a good choice as your central lab partner. Explore our flexible approach, global capabilities, and advanced diagnostics to optimize efficiency and enhance your research efforts.
Laboratory Documentation
The clinical trial journey begins with an in-depth consultation where our team collaborates closely with yours to gain a comprehensive understanding of your trial’s objectives, timelines, and specific requirements. We thoroughly review the trial protocol, identifying critical laboratory parameters, sample types, and testing methodologies required for success ensuring the highest standards of quality and accuracy.
Study-customized Documents
Analytical Plan
Detailed blueprint outlining trial objectives, methodologies, and analytical techniques, ensuring precise adherence to regulatory standards for the highest data integrity.
Our team of experienced scientists and clinical trial experts prepare a comprehensive Scope of Work, often referred to as the Analytical Plan collaborating closely with your project management team. This document outlines the specific objectives, methodologies, and analytical techniques to be employed during the trial. It serves as the blueprint for laboratory operations, ensuring that all testing and data collection are conducted according to your trial’s specific requirements. The Scope of Work is precisely crafted to meet regulatory standards and industry best practices, guaranteeing the highest level of data integrity.
Laboratory Testing
Leveraging our extensive experience in clinical trials, we design a laboratory setup tailored to the unique needs of your study. This includes selecting the appropriate testing facility, equipment, and methods to respect the required timelines and effective turn-around time to prepare most cost-conscious offer and ensuring at the same time compliance with industry standards and regulatory guidelines.
Study-customized Documents
Laboratory Manual
Step-by-step protocols, safety guidelines, and troubleshooting procedures, enabling site staff to perform tasks accurately and efficiently while maintaining compliance with regulatory guidelines.
The Laboratory Manual is the cornerstone of our commitment to standardized procedures and data consistency. It details step-by-step protocols for sample collection, handling, processing, and analysis, ensuring that every study site personnel follows standardized procedures. The manual includes comprehensive instructions, safety guidelines, quality control measures, and troubleshooting procedures. It is designed to be user-friendly yet thorough, enabling site staff to perform tasks accurately and efficiently while adhering to regulatory guidelines.
Samples Management
We work with you to develop a robust sample management strategy. This encompasses everything from sample collection kits and labeling protocols to shipment and storage of samples. Our goal is to maximize sample integrity and traceability throughout the trial’s lifecycle.
Study-customized Documents
Requisition Forms
They streamline sample collection with preprinted site information, pseudo-anonymized patient demographics, and barcoding for traceability, minimizing errors and enhancing overall trial efficiency.
Our customized requisition forms are carefully designed to streamline the sample collection process. These forms are tailored to your trial’s specific needs, including site preprinted information, patient demographics (pseudo anonymized), trial visits according to the schedule of assessment, sample types, and required tests as well as brief description on how to handle these samples at site facility until they are picked up. They are pre-labelled with unique barcoding and tracking features to ensure sample traceability and data accuracy. By simplifying the sample requisition process, we minimize the potential for errors and enhance the overall efficiency of your trial.
Study-customized Documents
Data Clarification Forms
Crucial for data consistency. Systematically address uncertainties in laboratory analysis, enhancing trial findings’ credibility and upholding patient safety by ensuring reliable data for critical treatment decisions.
Clarification documents serve as a crucial tool to ensure data consistency and traceability within the realm of laboratory analysis. These documents provide a systematic means to address and document any uncertainties or questions that may arise during the laboratory process. By doing so, they not only safeguard data accuracy but also enhance the credibility of trial findings. Moreover, clarification documents play a pivotal role in upholding patient safety by ensuring that every data point is reliable, thus influencing critical treatment decisions for trial participants.
Study-customized Documents
Shipping Documents
Compliant with international regulations, include comprehensive information on sample contents, handling instructions, and customs declarations, ensuring optimal sample condition during transit to central labs or designated facilities.
We create shipping documents that comply with international regulations and customs requirements. These documents include comprehensive information on sample contents, handling instructions, temperature control specifications, and all necessary customs declarations. We work closely with courier partners to ensure that your samples reach our central lab for clinical trials or other designated facilities in optimal condition and within specified timelines.
Online tracking, management and reporting
Define your trial-specific parameters, track samples and check your lab results whenever and wherever you want. Use LabOne – our proprietary Clinical Trial Laboratory Management System that facilitate study process management.
Labone - Central Lab Web-Based CTLMS
Key Features and Functionalities:
Data Management
Ensuring compliance with international regulatory standards is necessary. Our team carefully examines your trial’s regulatory requirements and implements processes that align with Good Clinical Practice (GCP), Good Laboratory Practice (GLP), and Good Clinical Laboratory Practice (GCLP) guidelines. This guarantees the validity and acceptance of your trial data by regulatory authorities.
Study-customized Documents
Data Transfer Agreement (DTA)
A pivotal component for secure data handling. It is a legally binding document outlining data ownership, transfer methods, confidentiality, and regulatory adherence, ensuring responsible transfer of sensitive clinical trial data for transparency and trust among stakeholders.
This is a pivotal component of central lab services for clinical trials, serving to establish the framework for secure data handling, privacy protection, and compliance. This legally binding document outlines data ownership, transfer methods, confidentiality, access rights, and regulatory adherence. It ensures the secure and responsible transfer of sensitive clinical trial data between the central laboratory and the client, maintaining data integrity and privacy while facilitating transparency and trust among stakeholders throughout the clinical trial process.
Lab Kits Production
We excel in producing custom collection kits tailored to your trial’s specific needs. Once kits are assembled, we design logistics processes, including secure transportation and storage, to guarantee that they reach your trial sites worldwide in a timely and controlled manner.
Our collection kits stand out for the ease of sample collection, prioritizing patient comfort and maintaining sample integrity. We have two kits set depending on study-specific needs. Routine Kits ensure accurate and comfortable sample collection, and Specialized Kits for studies requiring specialized efficacy parameter testing (for efficacy parameters like PK, ADA, Biomarkers).
Safety/Routine Collection Kits
our safety/routine collection kits are accurately designed to simplify the collection of samples while ensuring patient comfort and sample integrity.
These kits include:
Specialized Kits for Efficacy Parameters
(PK, ADA, Biomarkers, etc.) for studies requiring specialized efficacy parameter testing, we go beyond delivering kits that meet the highest industry standards.
These kits may include:
We designed easy -to-use samples collection kits
to reduce the risk or pre-analytical errors and maintain the sample integrity during transport:
Laboratory Kits Monitoring
Project Management & Coordination
Our team provides comprehensive training and support to trial sites upon client request, ensuring that site staff are well-versed in sample collection, handling, and shipping procedures. This preanalytical step is crucial for maintaining the quality and consistency of the samples throughout the testing workflow in the trial.
Throughout the course of your trial, our project management team diligently monitors progress, overseeing all aspects of laboratory operations.
Clinical Trials Project Management
These activities are coordinated and reported through the Lead Project Manager. They are available to the clients and investigators throughout the whole course of the study. Project Managers ensure that your clinical trials run smoothly, effectively and cost-efficiently.
Supplies and Samples Logistics
We conduct a thorough risk assessment to identify potential challenges and risks in the study setup process. Our proactive approach allows us to develop mitigation strategies, ensuring that any issues are addressed swiftly and efficiently to minimize disruptions to your trial.
Efficient logistics management is at the core of our central lab for clinical trials services. From route optimization and secure distribution of kit and lab materials to real-time tracking and timely issue resolution. Our logistic team ensures a seamless and reliable process, providing you with complete confidence and assurance throughout your trial.
Logistics Management
Our logistics experts work closely with your team to develop a customized logistics plan that aligns with the specific requirements of your clinical trial.
This plan includes:
Sample Collection Supplies
In addition to collection kits, we provide all necessary sample collection supplies, including safety gear and shipping materials, to ensure that samples are collected safely and securely.
Sample Shipment and Tracking
We manage the entire sample shipment process, from pickup at trial sites to delivery at our central lab or any other designated destination.
This includes:
Timely Reporting and Issue Resolution
We provide timely reports on the status of sample shipments, as well as proactive issue resolution in case of any shipping challenges. Our goal is to keep you informed and address any issues swiftly.