Site Management Organisation
Sponsors Area
We can support you in any study thanks to our wide portfolio of Therapeutic Areas, protocol designs and wide access to patient through our Medicover Group Network. We undertake comprehensive research across phases I-IV, featuring a dedicated early-phase unit specializing in pioneering first-in-human studies.




Our own Clinical Trial Sites network
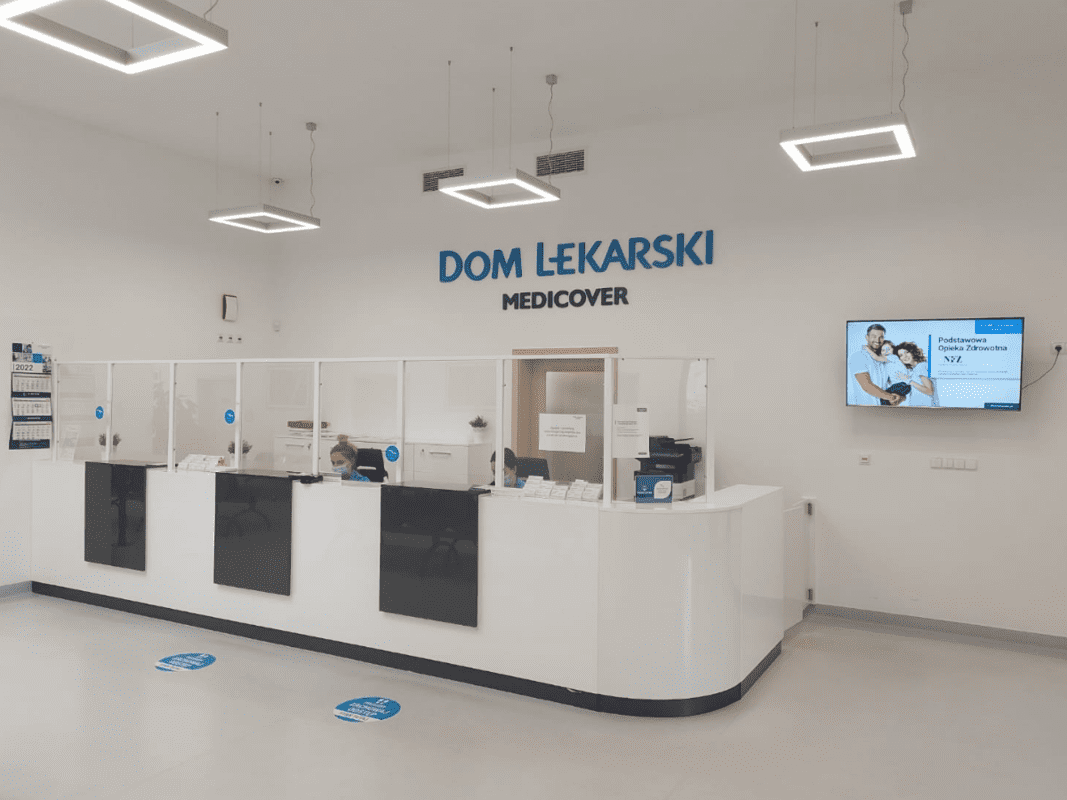
Our Site Management Organisation is network of proprietary owned Medical Centres in Poland. It is part of the Medicover Integrated Clinical Services (MICS) and Medicover Group. We benefit from this having a worldwide access to their infrastructure, proprietary owned laboratories, patients from almost any place in the world, clinical trials specialists, doctors as well as specialized equipment.
Toruń
MICS Medical Center
Bydgoszcz
MICS Medical Center
Szczecin
MICS Medical Center
Lubin
MICS Medical Center
Conducting clinical trials in many treatment disciplines
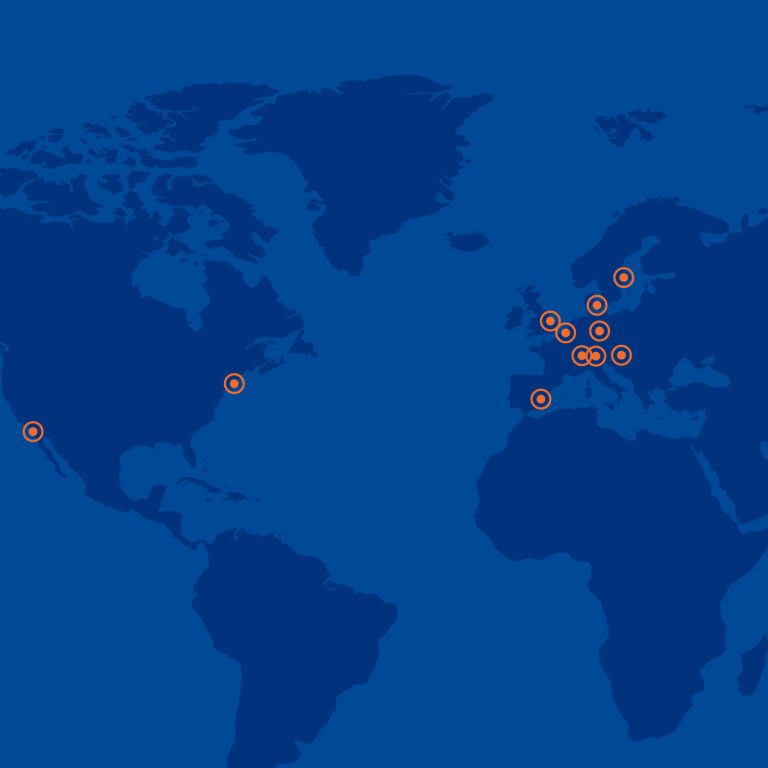
At MICS Site Management Organization, we cover a wide range of Therapeutic Areas. Our affiliation with the global Medicover Group provides us with the advantage of leveraging their facilities and engaging their staff. Currently the network is consisting of 178 medical centres, 40 hospitals, 52 pharmacies, 16 mental health centres, 30 fertility clinics, 113 dental clinics, 116 laboratories, 861 blood-drawing points (BDPs) and 28 clinics in 22 countries.
This collaboration improves our access to patients and allows us to implement innovative solutions that not only attract but also elevate care for both patients and medical professionals.
As a Medical Centers Network for innovative medicine, we excel in providing an optimal blend of sites, expertise, and patient access for each protocol, along with a seamless cooparation with local healthcare systems. Our capabilities extend to conducting first-in-human studies and managing Phase 2-4 studies, as well as specialized focus on oncology research.
Demonstrating a dedication to comprehensice assistance, our aim is to contribute to the successful completion of studies spanning diverse indications. Our approach is informed by the latest market research and RFIs, ensuring a guided and effective process.
Our statistics
Early Phase Studies
Comprehensive solutions in Early Phase studies, from document preparation to the final report.
Phase II – IV Clinical Studies
Our mixed site model offers you the best tailored solutions for your Phase II – IV Clinical Trials.
At MICS Site Management Organization, we cover a wide range of Therapeutic Areas. Our affiliation with the global Medicover Group provides us with the advantage of leveraging their facilities and engaging their staff. Currently the network is consisting of 178 medical centres, 40 hospitals, 52 pharmacies, 16 mental health centres, 30 fertility clinics, 113 dental clinics, 116 laboratories, 861 blood-drawing points (BDPs) and 28 clinics in 22 countries.
This collaboration improves our access to patients and allows us to implement innovative solutions that not only attract but also elevate care for both patients and medical professionals.
As a Medical Centers Network for innovative medicine, we excel in providing an optimal blend of sites, expertise, and patient access for each protocol, along with a seamless cooparation with local healthcare systems. Our capabilities extend to conducting first-in-human studies and managing Phase 2-4 studies, as well as specialized focus on oncology research.
Demonstrating a dedication to comprehensice assistance, our aim is to contribute to the successful completion of studies spanning diverse indications. Our approach is informed by the latest market research and RFIs, ensuring a guided and effective process.
Our statistics
Early Phase Studies
Comprehensive solutions in Early Phase studies, from document preparation to the final report.
Phase II – IV Clinical Studies
Our mixed site model offers you the best tailored solutions for your Phase II – IV Clinical Trials.
Why us?
Cross-border access to patients
from 22 countires mainly in Central and Eastern Europe and India.
Our Technology
brings hybrid and decentralized studies at your fingertips.
Centralized Service Model
from feasibility till contracting.
Access to innovative treatment options
Thanks to over 1000 successfully completed studies with high-quality care in the safest environment.
Access to innovative medicine, expertise, patients
as well as close collaboration with the local healthcare systems and Medicover Group entities.
Flexible approach toward the dynamically changing requirements
in the industry, including Remote Monitoring and Decentralized Studies.
Compliance with the highest international standards
High-quality services
In our daily activities we concentrate to increase Patients’ access to Clinical Trials in any therapeutical area, ensuring the safety of the trials’ participants. Achieving the highest medical standards requires strong clinical governance addressing the structures, systems and processes that ensure safety, quality, professional accountability and proper management in the operation and delivery of services.
To enable this approach, we have implemented a Clinical Quality Management System. Its components include systematic registration of certification of all clinical and diagnostic facilities; key performance and clinical quality reporting. The essential part of this system are Standard Operating Procedures (SOPs) that ensure high quality of services provided by our Medical Centers and standardize all processes.
Apart of that we systematically undergo many:
FDA inspections
EMA audits
Sponsors audits
Inspections are focused on the conduct of bioequivalence trial and Phase III study and confirmed the compliance with GCP, local regulations and the study protocol.
Contact
General inquiry
MARTA JEKA
Country Head SMO Poland
+48 500 403 916
marta.jeka@medicover.com