Solutions
Site Management Organisation
Where the patient-centric approach convert into stability of clinical trial process.





We have been providing bespoke services to our clients and their patients for over 25 years
Though all the years we have been making strategic use of Medicover assets: over 100 Medicover clinics, 100+ clinical laboratories, 2500+ global Medicover network partners and over 200 hospitals. We benefit from the vast expertise and enthusiasm of a workforce that has grown to more than 28,500 professionals around the world.
Therapeutic areas
We cooparate with numerous specialists from various medical fields, conducting clinical trials in 17 therapeutic areas such as Oncology, Hematology, Rheumatology, Neurology, Urology and many more. Additionally, our expertise extends to Rare Diseases, Orphan Drugs, Infectious Disease and Vaccine research, as well as studies in Cell and Gene Therapy, Virology, and Vaccine Laboratory.
Patients well-being
Our many years of experience in the field of clinical trials have allowed us to develop our own procedures – Standard Operating Procedures, ensuring the highest quality in maintaining accurate documentation of our actions. In our Site Management Organisation we are guided by the well-being of patients, ensuring them safety, and using the latest certified medical equipment.
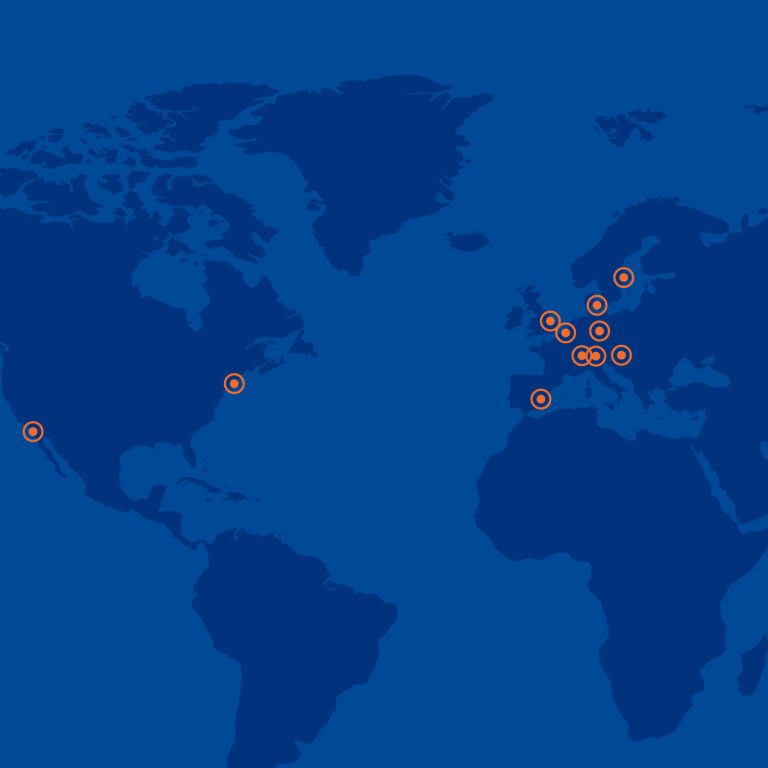
Global reach
We run clinical trials through our internal and external network in our core regions in EU, CEE and US. Our Diagnostic Services on-line channel reaches more than 2.1 million individuals per month, where we can find targeted patients for each clinical trial.
Quality
We conduct research projects in Phase I, II, III, and IV. All clinical trials are carried out in accordance with the principles of Good Clinical Practice – ICH GCP, applicable local regulations, and the requirements of study sponsors.
Our Site Management Organisation network
Over 700 successfully completed studies
Thanks to high quality and broad patient access.
State-of the art medical centers
The modern infrastructure equipped with certified medical equipment.
Average 1000 patients
currently enrolled to clinical trials.
Worked with 50+ Pharma Sponsors and CROs
Among them recognizible brands from pharmaceutical world.
Selected as a partner site and “Prime site”
For some of top 5 CROs.
More than 150 specialists
Thanks to ongoing global recruitment the number is still growing.
17 therapeutic areas
In which successfully recuit qualified patients for clinical trials.
More than 100 audits
including FDA, EMA, CEBK.
Discover our Medical Centres that enable patient access for most of therapeutic areas
Our Site Management Organization (SMO) are all about keeping up with the latest in medicine and working together to create new treatment methods. They cover clinical trials from phase I to IV, with a special focus on recruiting the right patients to each study. Our Medical Centers team up with specialists in various medical fields, expanding the range of research we conduct.
Find out more information about Site Management Organisation in your dedicated area
For Patients
For Doctors
For Sponsors
Contact
General inquiry
MARTA JEKA
Country Head SMO Poland
marta.jeka@medicover.com
+48 500 403 916