Clinical trials are a crucial component of the pharmaceutical and healthcare industries in developing new treatments and medications. However, the environmental and social impact of these trials has come under scrutiny in recent years, prompting the need for more sustainable practices. In this blog post, we will explore various approaches to elevate the environmental and social sustainability in clinical trials, with a specific focus on Medicover Integrated Clinical Services, as a vendor committed to sustainability and a signatory of the UN Global Compact under the cooperate policy.
The importance of sustainability approach in clinical trials
As the demand for innovative medical solutions grows, so does the environmental and social footprint of clinical trials. From resource-intensive processes to the ethical considerations of trial participants, the industry faces challenges that call for a shift towards more sustainable practices. Sustainable clinical trials not only align with global environmental and social goals but also contribute to the long-term success and reputation of pharmaceutical companies.
EU law mandates sustainability commitments
In line with the global push for sustainability, European Union (EU) laws have been enacted to ensure that vendors, including those involved in clinical trials, uphold sustainability commitments. These laws mandate vendors to report on their environmental and social impact, fostering transparency and accountability within the industry.
Medicover Integrated Clinical Services, as a vendor operating within the EU, adheres to these regulations, further reinforcing its dedication to sustainability. The company’s compliance with these laws reflects a broader industry trend towards prioritizing environmental and social responsibility.
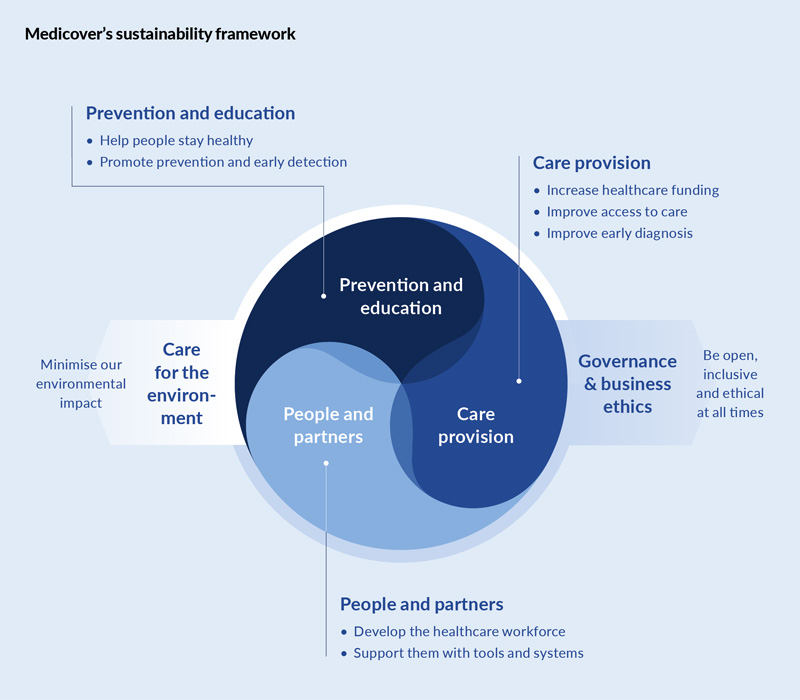
Medicover Integrated Clinical Services: vendor with a Sustainability Agenda
Medicover Integrated Clinical Services (MICS), a prominent player in the clinical trial services landscape, has demonstrated a strong commitment to sustainability. The company has implemented various initiatives to minimize its environmental impact and enhance social responsibility. These initiatives include waste reduction, energy efficiency, and many more in development.
MICS stands out as a vendor with a comprehensive sustainability agenda that encompasses the entire lifecycle of clinical trials. From the initial planning stages where we take the optimization approach to the final data analysis, the company integrates sustainable practices to reduce its carbon footprint and promote ethical conduct.
“To be able to demonstrate such a high positive net impact, and that 87% of our revenue is aligned with UN SDG #3 makes us very proud.”
Fredrik Rågmark, Medicover CEO
UN Global Compact signatory
One of the key indicators of our commitment to sustainability is Medicover status as a signatory of the United Nations Global Compact. By joining this voluntary initiative, the company pledges to align its operations and strategies with ten universally accepted principles in the areas of human rights, labor, environment, and anti-corruption. This commitment extends to all aspects of MICS’s services, including its involvement in clinical trials.
The last important event in this area was the 28th UN Climate Change Conference (COP 28). COP28 hosted the first-ever Health Day on 3 December, with more than 40 million health professionals joining the World Health Organization’s (WHO) call for health to be prioritized amid climate negotiations. In addition, the EU countries participated in this action as a Framework Party available in the climate change regime.
I’ve had the opportunity to participate in UN climate negotiations in previous years. The conclusion is clear: the journey towards more sustainable clinical trials is a collective effort. The importance of action in the healthcare system was also emphasized during the past UN climate negotiations COP28 in 2023. The group of 123 countries signed the COP28 UAE Declaration on Climate and Health, which highlights „the severe health implications of climate change”, and the urgent need for governments to prepare healthcare systems to cope with climate-related impacts such as extreme heat, air pollution and infectious diseases.

Sustainability in Clinical Trials: anticipating heightened emphasis
Elevating the environmental and social sustainability of clinical trials is a multifaceted endeavor that requires collaboration from all stakeholders. MICS serves as a prime example of a vendor actively contributing to this cause by incorporating sustainability into its operations, aligning with the principles of the UN Global Compact, and complying with EU laws.
As the pharmaceutical and healthcare industries continue to evolve, the emphasis on sustainability in clinical trials will only intensify. Vendors like Medicover Integrated Clinical Services set a positive precedent for the industry, demonstrating that a commitment to environmental and social responsibility is not only necessary for compliance but also essential for building a resilient and reputable future for clinical research.
Medicover, a proud signatory of the UN Global Compact Network, continues working towards the goals in responsible practices.
Author of this article
Izabela Juszko, Business Development Manager at Medicover Integrated Clinical Services
Medicover Integrated Clinical Services
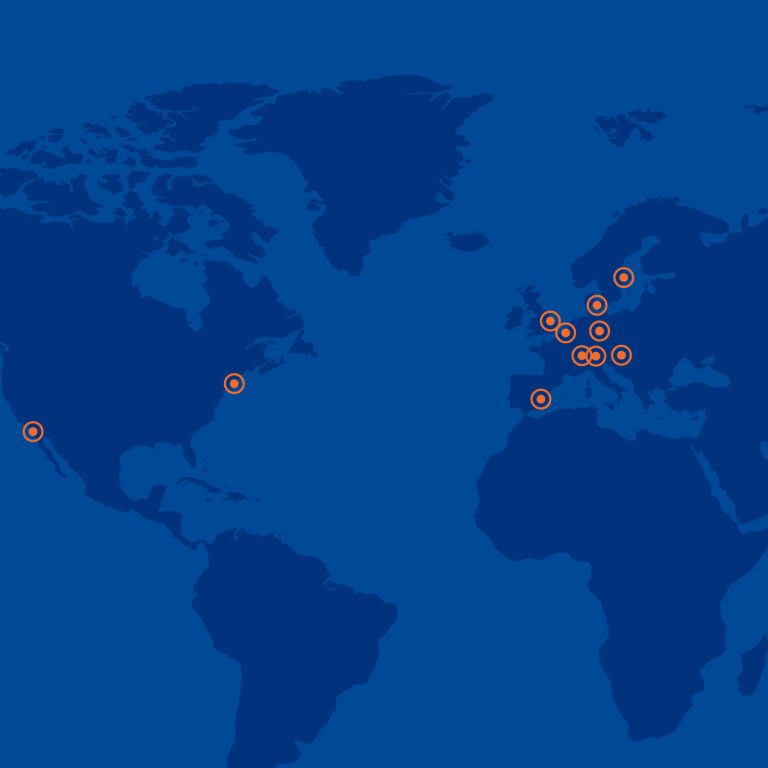
With Medicover’s strong presence in 13 countries across Central and Eastern Europe, we are uniquely positioned to support clinical trial needs. Our integrated approach covers comprehensive laboratory services and patient recruitment through our Site Management Organization. We offer an expansive portfolio of over 8000 laboratory parameters, including PK, PD, genomics, genetics, biomarkers, and PBMC isolation, catering to the diverse needs of your studies.